Which Statement Best Describes Alpha Decay
Which statement best describes what happens in radioactive decay. Because this energy must be shared between these two particles and because the alpha particle and daughter nucleus must have equal and opposite momenta the emitted alpha particle and recoiling nucleus will each.
Which equation represents alpha decay.

. An unstable nucleus releases an electron. 1 point After the ejection of an alpha particle the remaining nucleus has a mass number that is two less and an atomic number that is two less so alpha decay is a type of nuclear fission. An unstable nucleus releases a particle containing two protons and two neutrons.
An unstable nucleus rearranges its particles and releases energy. Which statement best describes the process of Alpha and Beta Decay. Which statement best describes the reaction.
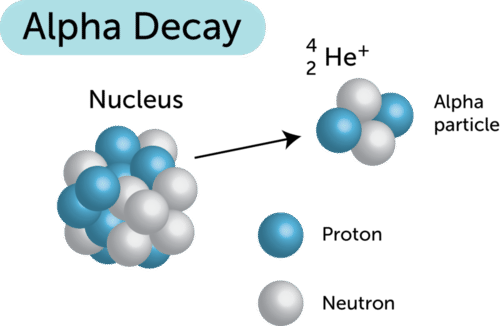
After the ejection of an alpha particle the remaining nucleus has a mass number that is two less and an atomic number that is two less so alpha decay is a type of nuclear fission. The nucleus can be of a different element than the original. Alpha decay An alpha particle is equivalent to the nucleus of an atom of which element1 point carbon helium nitrogen hydrogen Which statement best describes alpha decay1 point After the ejection of an alpha particle the remaining nucleus has a mass number that is four less and an atomic number that is two less so alpha decay is a type of nuclear fission.
Pick 2 1 Point In Alpha Decay the atom gains 2 protons and 2 neutrons In Alpha Decay the atom loses 2 protons and 2 neutrons. The resulting nucleus is more stable than the original nucleus. In Beta Decay one of the atoms neutrons splits into a proton and electron.
10Atoms of I-131 spontaneously decay when the AIt has a mass of 1 and a charge of 1. Which statement best describes the effect of radioactive decay on a nucleus. What can form as a result of a chemical reaction.
Use the periodic table link from the tools bar to answer the question. Uranium-233 undergoes alpha decay and then the daughter isotope undergoes another alpha decay. Aalpha particle Bbeta particle Cgamma radiation Dpositron.
Heres the answers for the quick check I took 1. When boron-10 is struck by neutrons it produces lithium-7 and an alpha. An unstable nucleus releases an electron.
In alpha decay alpha particles are ejected from the nucleus. DIt has a mass of 4 and a charge of 2. No it is not because the mass number does not change.
The electron cloud emits particles andor energy. In Beta Decay one of the atoms protons and electrons combine to form a neutron. Which statement best describes alpha decay.
1 point The resulting nucleus is more stable than the original nucleus. The nucleus must be of the same element as. The nucleus emits particles andor energy.
BIt has a mass of 0 and a charge of 1. Which statement best describes the process of alpha decay. The nucleus emits particles andor energy.
An unstable nucleus releases a single positively charged particle. Which statement best describes gamma radiation. The nucleus transfers particles andor energy to the electron cloud.
After the ejection of an alpha particle the remaining nucleus has a mass number that is two less and an atomic. In the alpha decay of a nucleus the change in binding energy appears as the kinetic energy of the alpha particle and the daughter nucleus. Which reaction describes an alpha emission.
Which statement best describes alpha decay. 1 point After the ejection of an alpha particle the remaining nucleus has a mass number that is two less and an atomic number that is two less so alpha decay is a type of nuclear fission. Which of the following statements about the nucleus is NOT true.
Which statement best describes the two reactions. Which statement best describes alpha decay. CIt has a mass of 0 and a charge of 0.
The model shows alpha decay which is a type of nuclear fission. Please help chemistry. A alpha beta and gamma B beta gamma and alpha C gamma alpha and beta B neutralization C saponification D transmutation D gamma beta and alpha 18.
Which statement best describes what happens in radioactive decay. Which statement best describes alpha decay. The electron cloud transfers energy to the nucleus.
Alpha Particle Emission Quick Check. 88226Ra 86222Rn 24He. After the ejection of an alpha particle the remaining nucleus has a mass number.
Two protons and two neutrons 3. A 24195Am - 23793Np 42He. 11Which statement best describes gamma radiation.
Which equation correctly describes this decay series. An unstable nucleus releases a particle containing two protons and two neutrons. After the ejection of an alpha particle the remaining nucleus has a mass number that is four less and an atomic number that is two less so alpha decay is a type of nuclear fission.
A mixture of emanations from radioactive atoms is passed through electrically charged plates as shown in the diagram below. Which statement best describes what happens in radioactive decay. Which statement best describes the process of alpha decayAn unstable nucleus rearranges its particles and releases energy.

Alpha Decay Definition Mechanism Types Uses And Examples

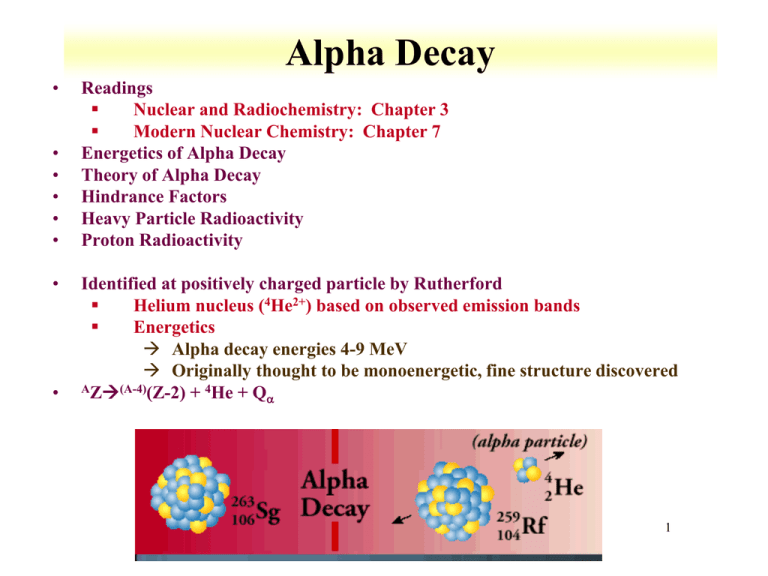
1 Alpha Decay Energetics Of Alpha Decay Theory Of Alpha Decay Hindrance Factors Heavy Particle Radioactivity Proton Radioactivity Identified At Positively Ppt Download

Comments
Post a Comment